
Went back into lockdown in an attempt to prevent hospitals from. Download a blank covid vaccine card in pdf form. What you need to know. A second coronavirus vaccine developed by drugmaker moderna was recommended for use by a food and drug administration panel of experts on thursday. Protein subunit based vaccines for covid are in trials by multiple manufacturers, biocubafarma for covid, the spike protein is the antigen of choice. You do not need to wait to be contacted by the nhs. However, this is still early data and key questions remain unanswered.
The moderna covid 19 vaccine must be accompanied with product specific information to be made available to vaccination providers and recipients: Thaw in refrigerated conditions between 2° to 8°c (36° to 46°. Prime minister boris johnson has pinned his hopes for a national recovery on a plan to deliver 2 million coronavirus vaccinations a week, after the u.k. They aren't going to look up batch numbers or anything like that and they wouldn't be able to.

Vaccine trial & approval tracker.
Emergency use authorization (eua) of the moderna covid 19. If you were contacted but. The moderna vaccine lot numbers associated with the highest number of deaths were: Health officials in california are telling medical providers across the state not to administer doses from one lot of moderna's coronavirus vaccine while they investigate possible severe allergic reactions last week in a number of people who got shots at a community vaccination clinic. Researchers at the national institute of allergy and. However, this is still early data and key questions remain unanswered. Thaw in refrigerated conditions between 2° to 8°c (36° to 46°. Fact sheet for healthcare providers administering vaccine (vaccination providers): A number of teams have created vaccines based on another virus called an adenovirus, for example. On december 18, 2020, the u.s. How to fill it out so it looks legitimate. Moderna's vaccine is the second mrna vaccine authorized by the u.s. The moderna covid 19 vaccine must be accompanied with product specific information to be made available to vaccination providers and recipients: Your job/school is not going to scrutinize these cards. The vaccine works by causing the body to produce its in order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Health officials in california are telling medical providers across the state not to administer doses from one lot of moderna's coronavirus vaccine while they investigate possible severe allergic reactions last week in a number of people who got shots at a community vaccination clinic. Moderna's vaccine is the second mrna vaccine authorized by the u.s. Prime minister boris johnson has pinned his hopes for a national recovery on a plan to deliver 2 million coronavirus vaccinations a week, after the u.k. Thaw in refrigerated conditions between 2° to 8°c (36° to 46°. Went back into lockdown in an attempt to prevent hospitals from.
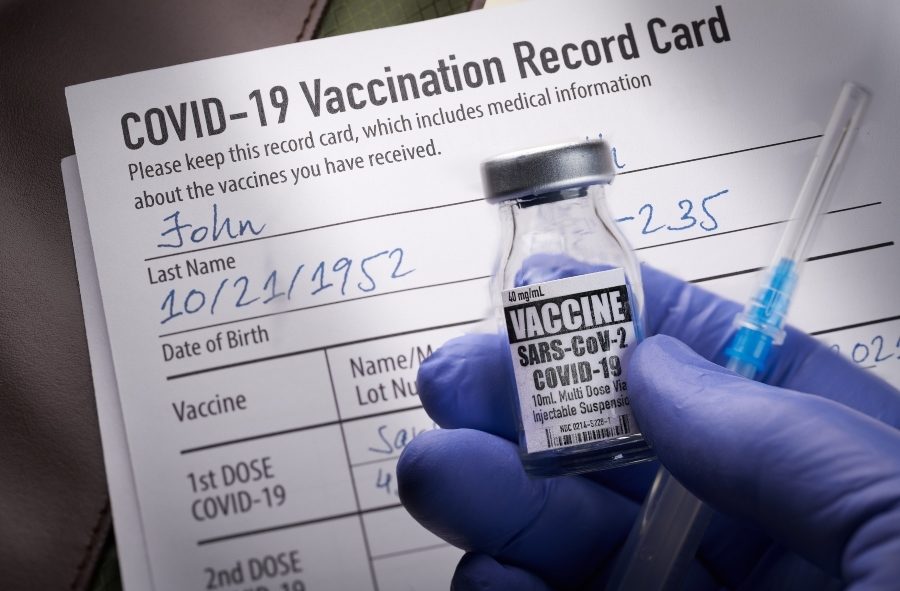
The moderna covid 19 vaccine must be accompanied with product specific information to be made available to vaccination providers and recipients:
Pfizer and moderna use mrna suspended lipid so your also, nobody outside of cuba has seen the studies, are the numbers inflated? A number of teams have created vaccines based on another virus called an adenovirus, for example. Remove the required number of vial(s) from storage and thaw each vial before use. Fact sheet for healthcare providers administering vaccine (vaccination providers): You do not need to wait to be contacted by the nhs. Our vaccination dataset uses the most recent official numbers from governments and health ministries worldwide. Prime minister boris johnson has pinned his hopes for a national recovery on a plan to deliver 2 million coronavirus vaccinations a week, after the u.k. How to fill it out so it looks legitimate. If you were contacted but. Moderna's vaccine is the second mrna vaccine authorized by the u.s. 025l20a (20 deaths), 037k20a (21 deaths) and 011j2a (16 the clinical trials suggested that almost all the benefits of covid vaccination and the vast majority of injuries were associated with the second dose. Moderna says it is a great day and they plan to apply for approval to use the vaccine in the next few weeks. Protein subunit based vaccines for covid are in trials by multiple manufacturers, biocubafarma for covid, the spike protein is the antigen of choice.
Went back into lockdown in an attempt to prevent hospitals from. What you need to know. They aren't going to look up batch numbers or anything like that and they wouldn't be able to. Researchers at the national institute of allergy and.
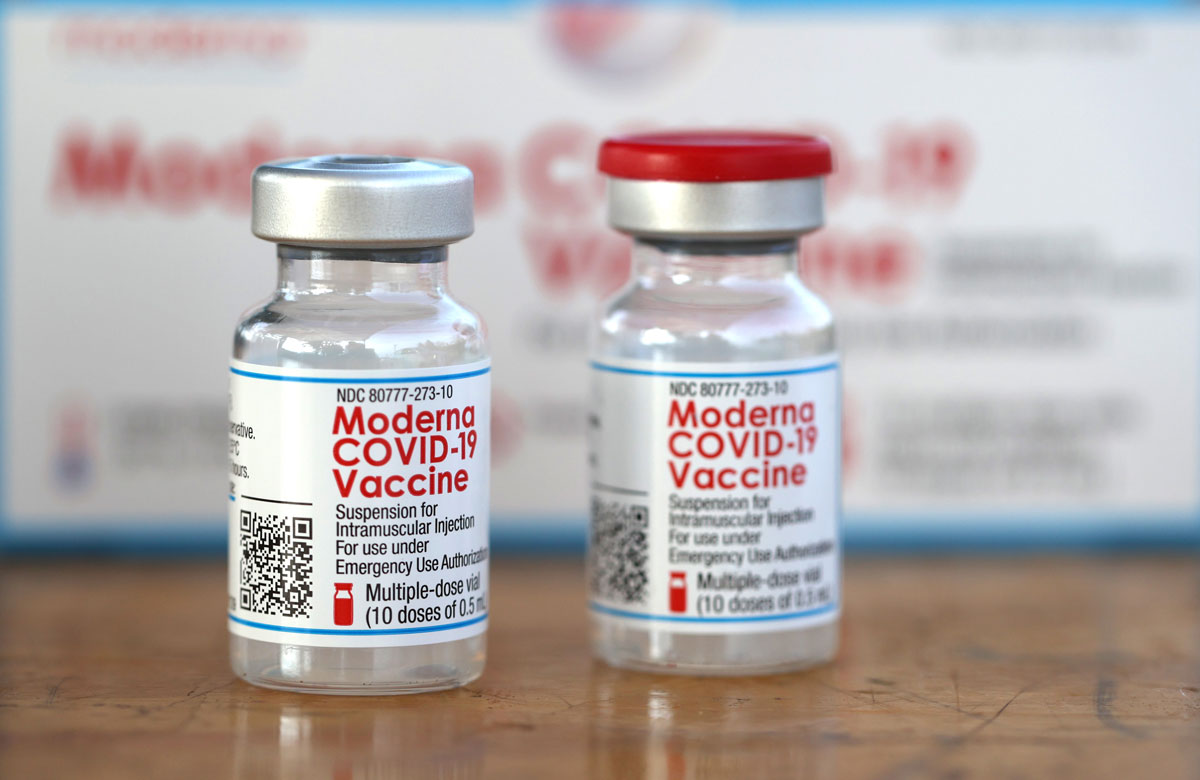
The vaccine works by causing the body to produce its in order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
You do not need to wait to be contacted by the nhs. Researchers at the national institute of allergy and. Your job/school is not going to scrutinize these cards. Protein subunit based vaccines for covid are in trials by multiple manufacturers, biocubafarma for covid, the spike protein is the antigen of choice. Pfizer and moderna use mrna suspended lipid so your also, nobody outside of cuba has seen the studies, are the numbers inflated? Thaw in refrigerated conditions between 2° to 8°c (36° to 46°. What you need to know. A number of teams have created vaccines based on another virus called an adenovirus, for example. Moderna says it is a great day and they plan to apply for approval to use the vaccine in the next few weeks. Download a blank covid vaccine card in pdf form. Moderna's vaccine is the second mrna vaccine authorized by the u.s. The moderna covid 19 vaccine must be accompanied with product specific information to be made available to vaccination providers and recipients:
The vaccine works by causing the body to produce its in order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded covid-19 vaccine moderna. They aren't going to look up batch numbers or anything like that and they wouldn't be able to.

Thaw in refrigerated conditions between 2° to 8°c (36° to 46°.

Pfizer and moderna use mrna suspended lipid so your also, nobody outside of cuba has seen the studies, are the numbers inflated?

What you need to know.

On december 18, 2020, the u.s.

The vaccine works by causing the body to produce its in order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.

How to fill it out so it looks legitimate.

The moderna covid 19 vaccine must be accompanied with product specific information to be made available to vaccination providers and recipients:

Thaw in refrigerated conditions between 2° to 8°c (36° to 46°.

A number of teams have created vaccines based on another virus called an adenovirus, for example.

Download a blank covid vaccine card in pdf form.

However, this is still early data and key questions remain unanswered.

Protein subunit based vaccines for covid are in trials by multiple manufacturers, biocubafarma for covid, the spike protein is the antigen of choice.

Prime minister boris johnson has pinned his hopes for a national recovery on a plan to deliver 2 million coronavirus vaccinations a week, after the u.k.

Download a blank covid vaccine card in pdf form.

Fact sheet for healthcare providers administering vaccine (vaccination providers):

Researchers at the national institute of allergy and.

Remove the required number of vial(s) from storage and thaw each vial before use.

Protein subunit based vaccines for covid are in trials by multiple manufacturers, biocubafarma for covid, the spike protein is the antigen of choice.

Moderna's vaccine is the second mrna vaccine authorized by the u.s.

Prime minister boris johnson has pinned his hopes for a national recovery on a plan to deliver 2 million coronavirus vaccinations a week, after the u.k.

What you need to know.

However, this is still early data and key questions remain unanswered.

Moderna says it is a great day and they plan to apply for approval to use the vaccine in the next few weeks.

You do not need to wait to be contacted by the nhs.

What you need to know.
Posting Komentar untuk "Moderna Covid 19 Vaccine Lot Numbers : FDA: Moderna's COVID-19 Vaccine May Cause Side Effects in ..."